Lecture by Phillip A. Horwitz, MD
July 2020

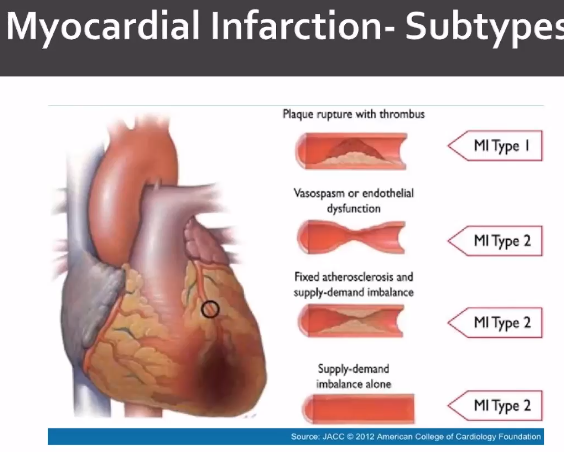
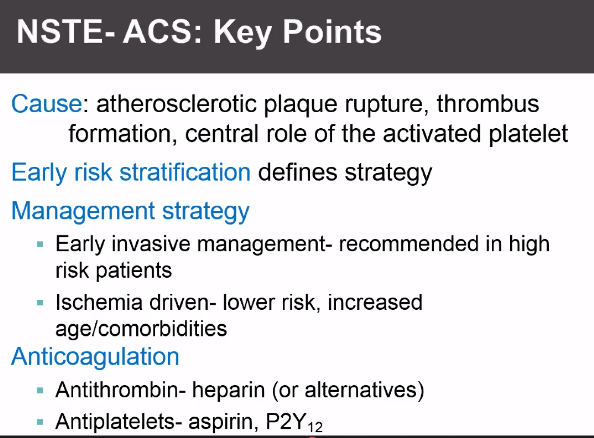
All forms of ACS (unstable angina, NSTEMI, and STEMI) are all due to reduction in coronary blood flow
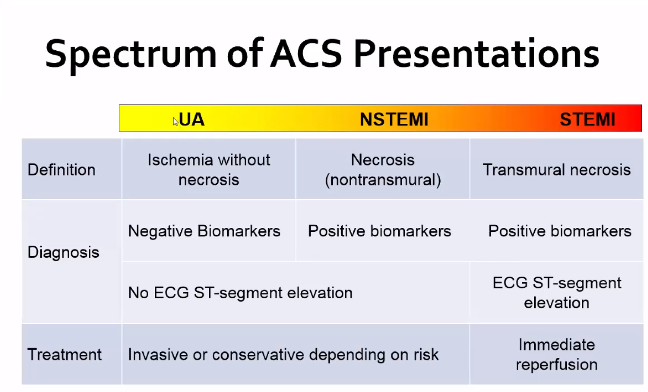
Incredibly common diagnosis. 1.6 million in US annually – Roughly 50% have UA and the other 50% have MI
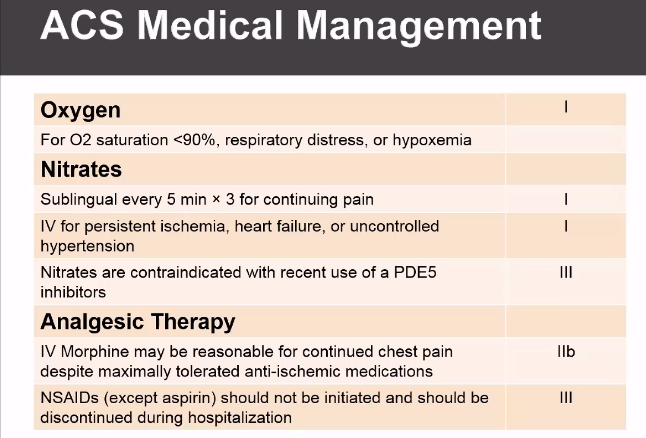
Standard therapy, they present to ED with chest pain. They get vitals taken, IV put in, oxygen applied (though some recent data shows that over oxygenation may be harmful, certainly should keep >90%).
They are then stratified based on the EKG findings. If no ST changes, will stratify based on troponin elevation
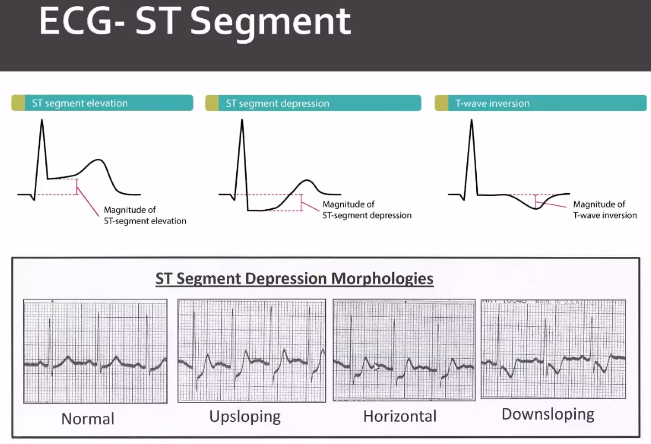
Flat and down sloping are more specific for ischemia.
Non-Ischemic ST depression: Ventricular hypertrophy, intraventricular conduction delay, digoxin, hypokalemia, hypothermia, CNS (SAH/Stroke,seizure), Physiologic (tachycardia or hyperventilation)
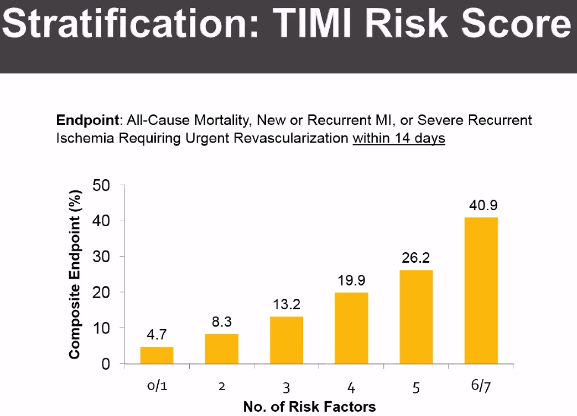
Many score systems are in place to stratify risk Score: TIMI, etc. Level of 0-2 is considered low risk for TIMI score.
MANAGEMENT STRATEGIES
NSTEMI
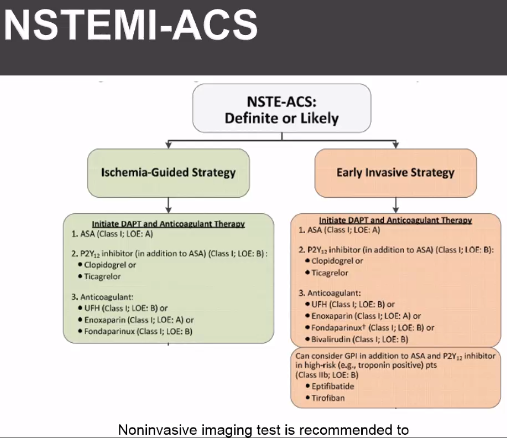
Early invasive – angiography <24-48 hours. Give anticoagulation and anti-ischemics
Ischemia driven – Anticoagulation, non-invasive risk strategies. Angiography for recurrent ACS symptoms or high risk on stress testing
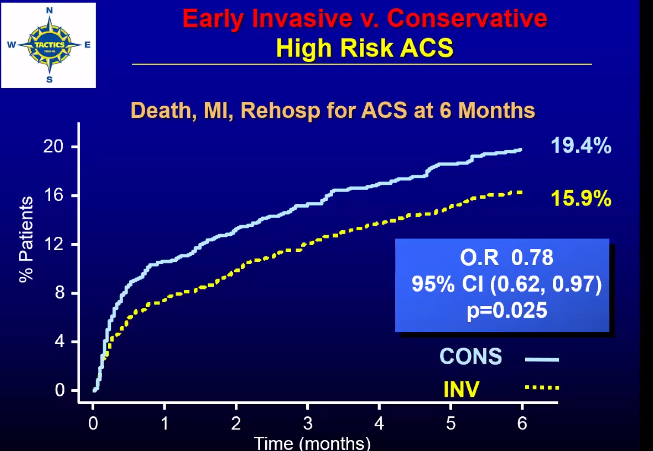
Tactics trial https://pubmed.ncbi.nlm.nih.gov/11419424/
Unlike patients presenting with STEMI in which there is a clear mortality benefit to emergent coronary angiography and PCI, it is less clear whether patients with non-STEMI ACS (unstable angina or non-ST segment elevation MI) also benefit from routine early angiography and intervention.
The 2001 Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy – Thrombolysis in Myocardial Infarction 18 (TACTICS-TIMI 18) trial randomized 2220 patients to either a protocolized early invasive strategy versus a delayed selectively invasive strategy with each arm receiving the IIb/IIIa inhibitor tirofiban. In the early invasive strategy arm, 97% of patients underwent coronary angiography a median of 22 hours (all within 48 hours) after presentation with PCI or CABG to culprit lesions. In the conservative strategy, patients underwent coronary angiography only if noninvasive stress testing was positive or there was failure of medical therapy (prolonged angina at rest, hemodynamic instability, recurrent angina or MI). Overall, 60% of patients in the early invasive arm underwent revascularization versus 36% in the conservative arm. TACTICS-TIMI 18 demonstrated a clear reduction in major adverse cardiovascular events with an early invasive approach, driven primarily by a reduction in nonfatal MI and recurrent ischemia. This benefit was most apparent in patients presenting with ST segment changes, troponin elevation at presentation, and patients with intermediate or high TIMI risk scores (3 or greater). Notably there was no mortality benefit with an early invasive approach, and this strategy was associated with a 2% absolute increase in protocol-defined bleeding. Importantly, patients with TIMI score 0-2 and patients with undetectable troponins (25% of the study population) did not appear to benefit from routine angiography and PCI, suggesting that it is reasonable to pursue stress testing in these low-risk patients with intervention reserved for patients with significant ischemia on functional testing.
Overall the results of TACTICS-TIMI 18 provide practical guidance for the selection of patients who are most likely to benefit from early coronary angiography and intervention in non-STEMI ACS. It is important to note that although both the biomarker and TIMI risk stratification findings (i.e., patients with negative troponins or patients with TIMI risk score 0-2 do not benefit from an early invasive strategy) are often incorporated into routine clinical practice, the interaction between the primary outcome and these individual subgroups did not reach statistical significance; however, given stark numerical differences and apparent risk-response pattern within the TIMI score group (i.e., as TIMI risk increased, benefit with early angiography increased), lack of statistical interaction likely reflects underpowered subgroups in this case rather than a spurious finding. In addition, TACTICS-TIMI 18 was also a trial of the IIb/IIIa inhibitor tirofiban, which continues to have a limited role in NSTE-ACS, primarily as bailout therapy in patients undergoing PCI of high-risk lesions.
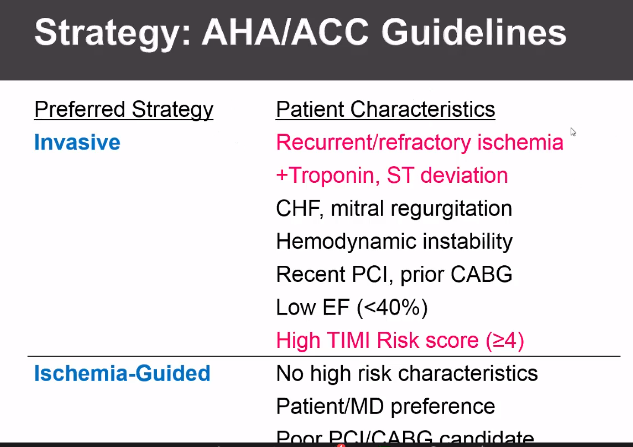
Angiography is preformed immediately for refractory angina, hemodynamic instability, ventricular arrythmia, or acute heart failure. Those without immediate need but still intermediate risk will undergo invasive strategy within 48 hours while receiving medical therapy. Medical therapy will usually include both anti-thrombin strategies and anti-platelet strategies seen below. ACC/AHA Class 1 recommendation: anti-coagulation recommended for all patients with NSTE-ACS: Unfractionated heparin or LMWH. Aspirin Class I therapy in all ACS patients 162-325 mg (rapidly inhibits platelet activation by halting thromboxane A2 production). P2Y12 inhibitor AHA/ACC Class 1 (clopidogrel, prasugrel, ticagrelor). Certain newer trials so benefit to Ticagrelor and prasugrel, still not clear about which is best but all patients need to be on one of the 3. Newer agents likely better based on recent studies. Prasugrel has increased bleeding risk but better ichemic outcomes. Ticagrelor (see PLATO Trial) has reduction in death from vascular causes or MI in 1 year compared to clopidogrel, may see bradycardia. Prasugrel and ticagrelor more rapid onset. Unclear if patients should be pre-treated prior to invasive strategies in first 24 hours. May have benefit, but may also delay CABG if needed.
PLACE ALL PATIENTS WITH NSTEMI ON APPROPRIATE MEDICAL THERAPY. RESIDENTS CAN ALWAYS START WITH ASPIRIN AND ANTI-THROMBIN AND DISCUSS PGY12 TIMING with CARDS.
https://pubmed.ncbi.nlm.nih.gov/11519503/
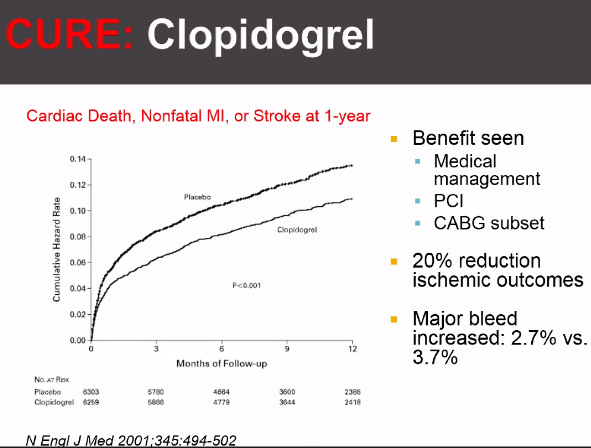
CURE TRIAL
Early trials such as the VA Cooperative Study (1983) and ISIS-2 (1988) demonstrated that aspirin, which inhibits platelets through irreversible cyclooxygenase blockade, decreases mortality in UA/NSTEMI. Clopidogrel, a newer antiplatelet agent that irreversibly binds to the P2Y12 ADP receptors, has been suggested to have better outcomes. CURE was designed to evaluate if clopidogrel would further improve outcomes from ACS when added to ASA.
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial randomized 12,562 patients with UA/NSTEMI to either dual antiplatelet therapy with aspirin plus plavix or to aspirin alone immediately upon presentation. At a mean follow-up of 9 months, dual antiplatelet therapy was associated with a significant reduction in the composite primary endpoint of CV mortality, nonfatal MI, or stroke, largely due to fewer MIs. However, the addition of clopidogrel was associated with increased rate of major bleeding.
PLATO Trial
The thienopyridines clopidogrel (CURE; 2001) and prasugrel (TRITON-TIMI 38; 2007) irreversibly inhibit platelets through P2Y12 ADP receptor blockade and reduce CV outcomes in ACS. The irreversible effects of both thienopyridines is thought to account for the increased bleeding from major surgical procedures including CABG, and thus investigators sought an antiplatelet agent with different pharmacokinetics. Ticagrelor is a nucleoside analogue acts via reversible inhibition of ADP receptors. It has a more rapid onset, more potent platelet inhibition, and more predictable pharmacodynamics, although its clinical utility had not yet been established.
The 2009 Platelet Inhibition and Patient Outcomes (PLATO) trial randomized 18,624 patients with ACS (37.5% with STEMI) to ticagrelor or clopidogrel, in addition to standard care. At 12 months, the ticagrelor group had lower rates of CV death, MI, or stroke than clopidogrel (9.8% vs. 11.7%) and all-cause mortality (4.5% vs. 5.9%). While there was no difference in major bleeding, ticagrelor was associated with more non-CABG-related bleeding (4.5% vs. 3.8%). The ticagrelor group experienced a higher rate of dyspnea (13.8% vs. 7.8%) and a trend towards more bradycardia (4.4% vs. 4.0%), likely due to ticagrelor’s close resemblance to adenosine.
STEMI

ST elevation is hallmark of disorder
Non-ischemic ST elevation: Early repolarization, LV hypertrophy, LBBB, peri-myocarditis, hyperkalemia, Brugada, ARVD, WPW, takotsubo’s.
TIME IS MUSCLE!
Treatment is emergent reperfusion:
Clinical syndrome of MI
ECG with new LBBB, or typical ST changes >1mm ST segment elevation in two leads or 1.5 mm in V2-V3
Thrombolytic therapy: treatment at rescue hospital <30 minutes if PCI >2 hours away. Some stroke risk. NSTEMI shows no benefit on subgroup analysis, only use for STEMI. Contraindicated with high bleeding risks, see checklist.
Primary PCI: Class 1 for STEMI <12 hour symptoms, contraindication to fibrinolytics, STEMI and shock or severe CHF. Class IIa, STEMI or clinical ACS between 12-24 hours. Goal within 90 minutes of first contact. Nearly everyone is eligible (>95%) Higher TIMI 3 flow rates, less risk of intracranial bleeding than fibrinolytics. Less recurrent ischemia. Downside is that it takes time, some risk of renal insufficiency with high contrast loads.

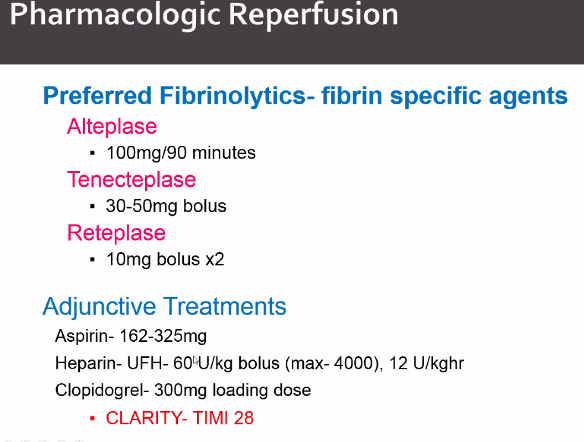
Everyone who gets lytics gets aspiring and heparin. Clopidogrel may also show benefit, see mentioned trial.

This drives the guidelines. Do PCI if able to get into
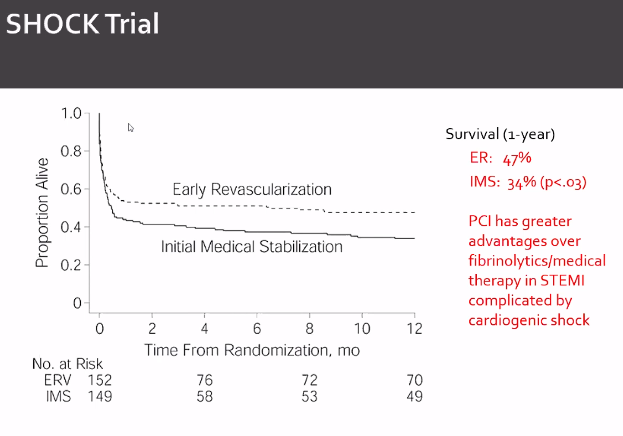
SHOCK Trial
The role of primary PCI in cardiogenic shock was evaluated in the 1999 SHould we emergently revascularize Occluded Coronaries for Cardiogenic shocK (SHOCK) trial, which randomized 302 patients in cardiogenic shock after acute MI to a strategy of early revascularization (PCI or CABG within 12 hours of diagnosis) or to initial medical stabilization including fibrinolysis and IABP. There was a trend towards improved survival in the primary endpoint of mortality at 30 days, but this failed to achieve significance. This may be due to the fact that the trial was underpowered to detect such a difference. However, the benefit became significant at 6 months, and follow-up of the SHOCK trial cohort demonstrated the benefit or revascularization at 1 and 6 years, suggesting that the benefit of revascularization persisted for years.
Subgroup analysis found that the benefit of revascularization on survival appeared to be limited to patients <75 years, but there were too few patients ≥75 years to make any solid conclusions regarding the magnitude of benefit or harm. Indeed, subsequent studies found that the same benefit extended to patients ≥75 years old.
ACS MEDICAL MANAGEMENT
Beta-adrenergic blockers: Initiate within first 24 hours in absence of heart failure and shock.
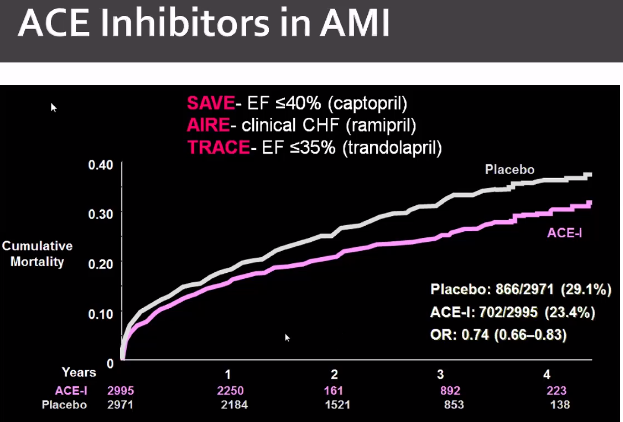
Renin-Angiotensin System inhibitor
Class I: ACEI wihtin 24 hours of anterior STEMI, CHF, EF<40%. ARB for ACEI intolerance. Aldosterone antagonist – STEMI patients on ACEI/BB and EF<40% with symptomatic CHF
Class II: Reasonable for all STEMI patients without contraindications. \
Lipid Management:
Class 1: high intensity statin therapy in all patients with STEMI
Age <75 high intensity, Age >75 moderate intensity. High (40-80 atorvastatin, 20-40 rosuvastatin)
Discharge
med list: Aspirin 81 mg indefinitely, PGY12, beta blocker, statin, Ace-I (DM, EF<40%, HTN, stable CKD), SL NTG
Refer to Phase II cardiac rehab, referral for smoking cessation, exercise program and lifestyle coaching.

Most people substitute aldosterone for cost
KEY POINTS
-
When patients with cardiovascular risk factors present with chest pain, the quality of symptoms, the age, and the sex of the patient can help to differentiate stable angina pectoris from other causes of chest pain.
-
Stress testing is most useful in patients at intermediate pretest likelihood of coronary artery disease (10% to 90%).
-
Aspirin or clopidogrel (if aspirin-allergic) is recommended in all patients with established coronary artery disease unless contraindicated; the use of newer antiplatelet agents (prasugrel, ticagrelor) as monotherapy has not been tested in patients with stable angina pectoris.
-
All patients with stable angina pectoris should receive a statin and a β-blocker.
-
ACE inhibitors are indicated in the treatment of stable angina pectoris, particularly in patients with concomitant diabetes mellitus and left ventricular systolic dysfunction.
-
Percutaneous coronary intervention improves angina symptoms and quality of life in patients with stable angina pectoris but does not increase survival or reduce future cardiovascular events.
-
For stable angina pectoris, percutaneous coronary intervention is reserved for patients with refractory symptoms while on optimal medical therapy, those who are unable to tolerate optimal medical therapy owing to side effects, or those with high-risk features on noninvasive imaging.
-
Clinical practice guidelines do not recommend the routine use of ECG monitoring, stress testing, or anatomic testing (coronary CT angiography or invasive angiography) in asymptomatic patients after percutaneous coronary intervention or coronary artery bypass graft surgery.
-
In patients with stable angina pectoris who undergo percutaneous coronary intervention, dual antiplatelet therapy (aspirin plus clopidogrel) is recommended for at least 1 month after bare metal stent implantation and at least 6 months after drug-eluting stent implantation.
-
In patients with ST-elevation myocardial infarction, when percutaneous coronary intervention cannot be readily achieved within 120 minutes, thrombolytic therapy is recommended in the absence of contraindications.
-
Patients with ST-elevation myocardial infarction who receive thrombolytic therapy should be transferred to a percutaneous coronary intervention–capable facility because of the potential for thrombolytic failure.
-
All patients who present with ischemic chest pain should be treated initially with aspirin, β-blockers, and nitrates; once the diagnosis of a non–ST-elevation acute coronary syndrome has been established, risk stratification can be used to guide the clinical use of additional therapies.
-
All patients with a non–ST-elevation acute coronary syndrome should receive a statin and dual antiplatelet therapy with aspirin and a P2Y12 inhibitor; in patients at intermediate or high risk, additional therapies (anticoagulant agents, glycoprotein IIb/IIIa inhibitor) should be considered.
-
Dual antiplatelet therapy is recommended for at least 1 year in all patients with a non–ST-elevation acute coronary syndrome, unless an increased risk of bleeding exists.
-
Once medical therapy has been initiated and risk stratification has occurred, guidelines recommend that low-risk patients with a non–ST-elevation acute coronary syndrome be treated conservatively and that intermediate- to high-risk patients be considered for early invasive treatment.
ADDITIONAL RESOURCES
We presented a case of a 60 year old male with history of HTN, HLD, GERD and PE who presented for intermittent chest pain for 3 days that he described as tightness and pressure over his sternum. It does radiate up to his neck and is made worse by exertion. Family history of MI in his father in his early 50s. His cath revealed 100% stenosis due to thrombotic lesion in the mid-Cx. He was discharged but returned 2 days later due to sudden severe shortness of breath, chest pressure and no improvement with nitroglycerin, he had a new harsh systolic murmur across his precordium and was diagnosed with new severe mitral regurgitation secondary to papillary muscle rupture. He was taken urgently to he cath lab after intra-aortic balloon pump placed for mitral valve replacement.
CaseConference10.29.2018Slides
Post by Roger D. Struble Jr. MD MPH
